Maynard Group Research Overview
Research in the Maynard Group lies at the frontiers of polymer chemistry and medicine. We are equally excited about fundamental science as we are about application in drug delivery. Currently the group is focusing on three main areas: (1) Conjugations and Conjugates; (2) Responsive Drugs, and (3) Bioderived Materials.
Conjugations and Conjugates
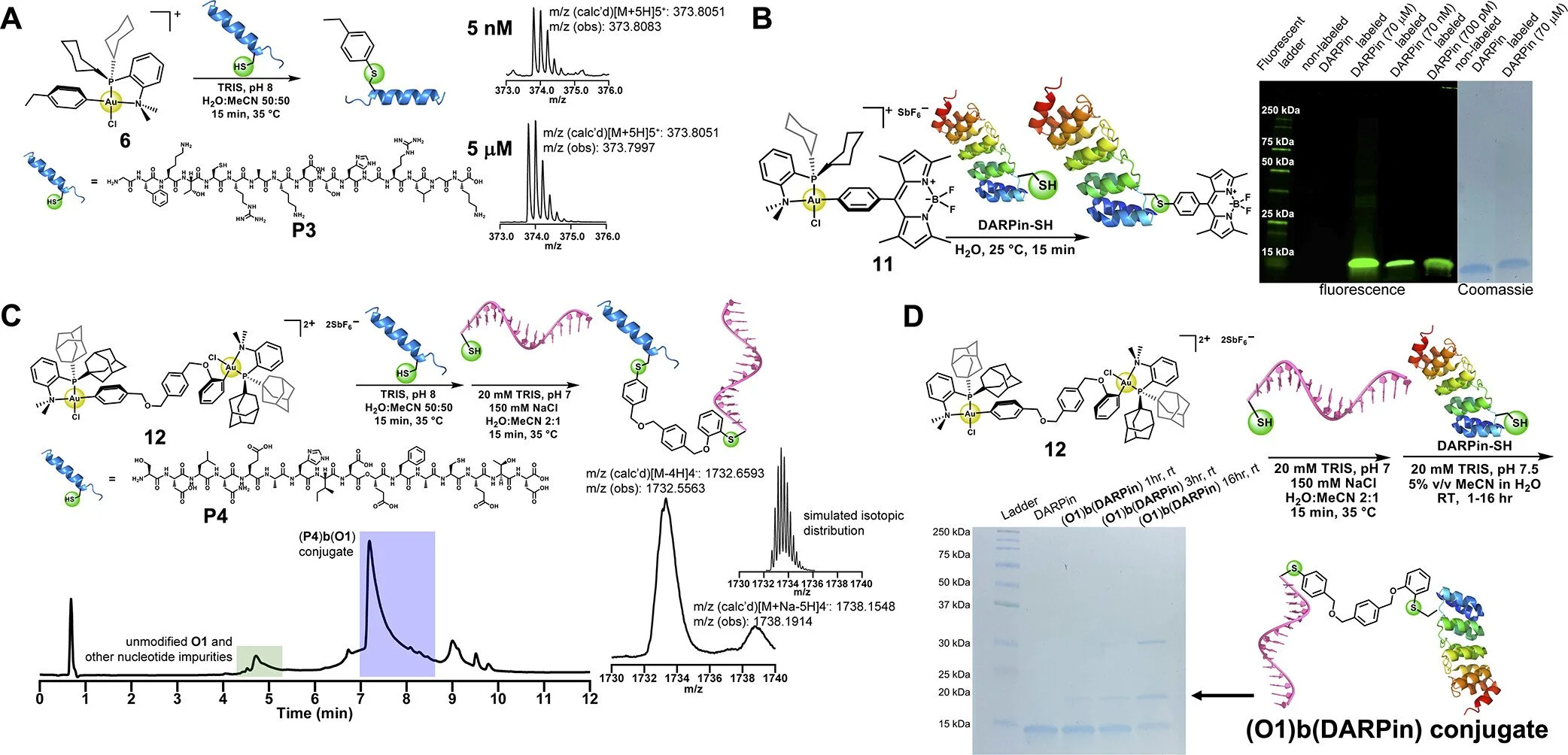
Protein conjugates are widely used in medicine; there are over thirty approved by the Federal Drug Administration. Our group has made extensive fundamental contributions to this field. For example, we showed that controlled radical polymerizations could be used to graft to and graft from proteins. We also created trehalose polymers and sulfonated polymers that stabilize the proteins to which they are attached to extreme storage conditions. Recent work has been focused on exploring gold(III) oxidative addition complexes to synthesize S-arylated biomolecules. These complexes are being exploited to prepare complex protein-polymer conjugates such as block conjugates and heteroconjungates. In addition, we are developing new antibody drug conjugate (ADC) linkers that are monodisperse and hydrophilic for high drug-to-antibody ratios.
Take a look at this paper for example:
Doud, E. A.; Tilden, J. A. R.; Treacy, J. W.; Chao, E. Y.; Montgomery, H. R.; Kunkel, G. E.; Olivares, E. J.; Adhami, N.; Kerr, T. A.; Chen, Y.; Rheingold, A. L.; Loo, J. A.; Frost, C. G.; Houk, K. N.; Maynard, H. D.; Spokoyny, A. M. “Ultrafast Au(III)-Mediates Arylation of Cysteine,” J. Am. Chem. Soc., 2024, 146, 12365-12374.
Responsive Drugs
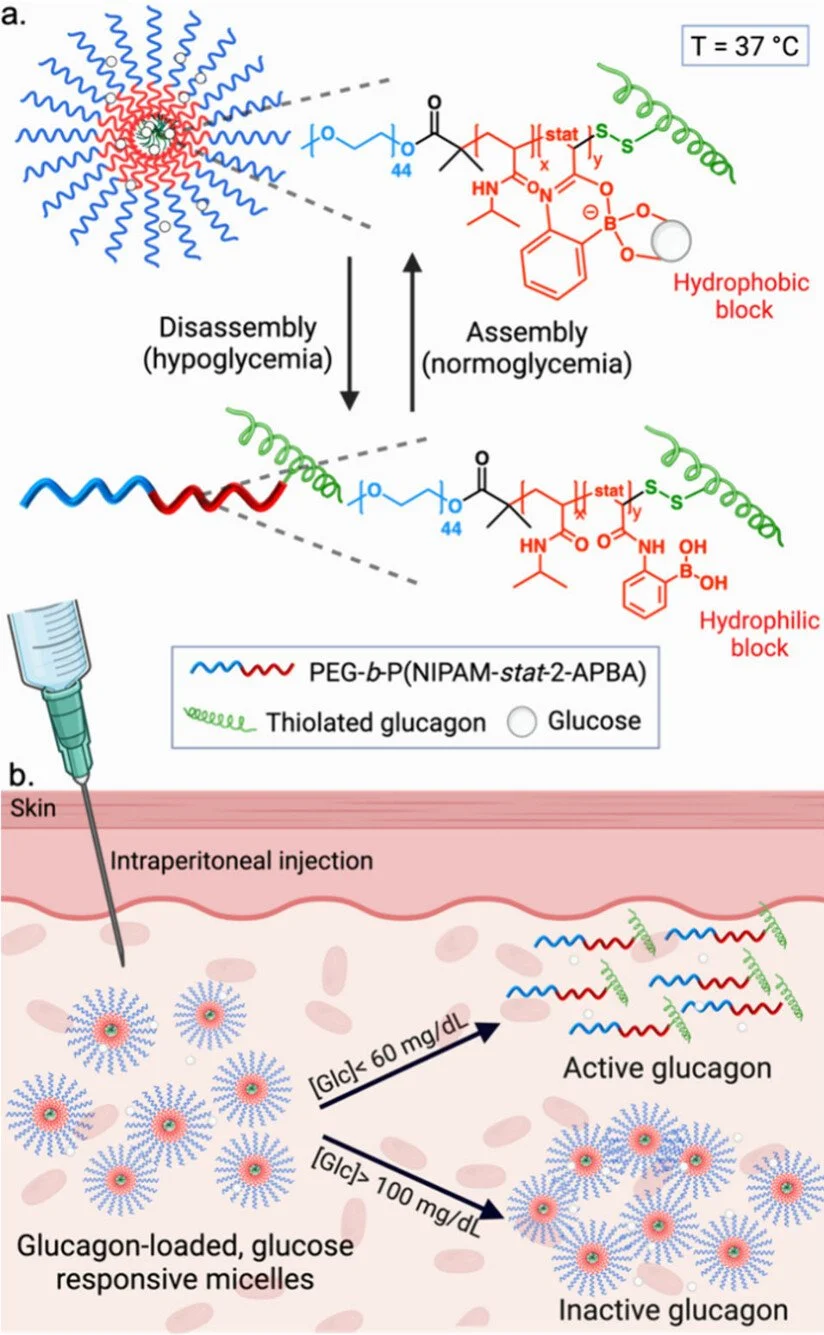
Materials that respond to release a drug only when and where needed in the body are called responsive drugs. These are important to prevent symptoms of a disease or or to lower off targeting effects. A major effort of the group is to prepare materials that sense low glucose levels to release the hypoglycemia drug glucagon. For example, block copolymers with 2-acrylamidophenylboronic acid units that change the lower critical solution temperature of poly(N-isopropylacrylamide) upon binding to glucose were synthesized such that at the counterregulatory sugar levels, glucagon is revealed. The micelles were shown to treat and prevent hypoglycemia in mice. This research at the interface of polymer science and medicine is ongoing. We also focus on fundamental problems within this field, for example, developing new self-immolative linkers.
Here's an example of this type of research:
Vinciguerra, D.; Sivasankaran, R. P.; Yang, J.; Georgiou, P. G.; Snell, K.; Pesenti, T.; Collins, J.; Tamboline, M.; Xu, S.; van Dam, R. M.; Messina, K. M. M.; Hevener, A. L.; Maynard, H. D. “A Glucose-Responsive Glucagon-Micelle for the Prevention of Hypoglycemia,” ACS Central Science, 2024, 10, 2036-2047.
Biomimetic and Bioderived Materials
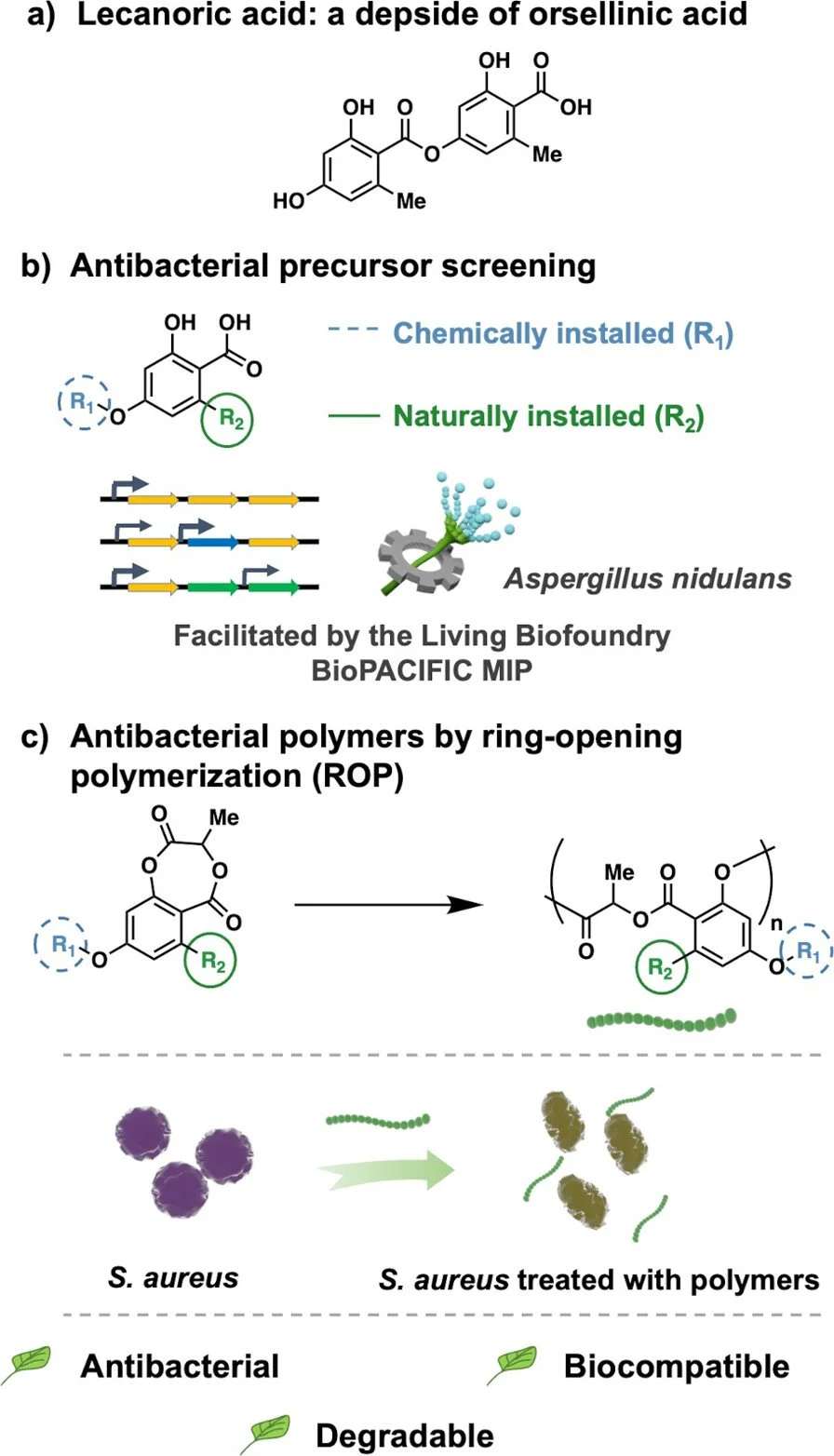
The BioPACIFIC Materials Innovation Platform is a NSF funded ecosystem that accelerates the synthesis of biomimetic and bioderived materials. This is accomplished through a combination of robotic monomer production from bacteria and fungi and high throughput polymer synthesis and characterization. The Maynard group is exploiting this to prepare antimicrobial polymers and materials that mimic natural molecules called depsides. In a recent example, hydroxybenzoic acids generated from fungi were chemically modified to produce monomers. Polymers were synthesized by ring opening polymerization to prepare depside mimics that proved to be antimicrobial against S. Aureus bacteria. Several of the polymers had excellent biocompatibility and disrupted biofilms, and all were degradable. Current work is focusing on creating additional depside mimics with predictable degradation rates as antibiotics.
Look at this paper to read more:
Salas-Ambrosio, P.; Vexler, S.; Sivasankaran, R. P.; Vlahakis, N.; Lai, R. S.; Johnson, C.; Baas-Maynard, S. I.; Min, D. S.; Lower, H.; Doyle, A. G.; Tang, Y.; Rodriguez, J. A.; Chen. I. A.; Read de Alaniz, J.; Maynard, H. D. “Biosourced Functional Hydroxybenzoate-co-Lactide Polymers with Antimicobial Activity,” JACS, 2025, 147, 19230-19238.